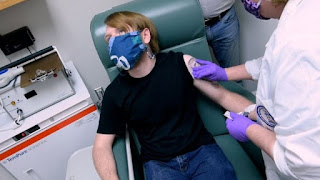
Pfizer and its partner bionetek filed Friday an application for an emergency permit in the United States to use the covid-19 vaccine.
The U.S. Food and Drug Administration to decide whether the vaccine is safe.
It is not yet clear how long it will take the FDA to study the data, but the U.S. government expects to approve the vaccine in the first half of December.
Data from an advanced trial showed that the vaccine protects 94 percentof adults over 65 years old.
And asked Britain already 40 million doses is supposed to get 10 million by the end of the year.
When can we expect to start the process of vaccination?
Pfizer and biontek said that if the FDA license comes in the first half of next month, they would be “ready to distribute the vaccine within hours.”
If the vaccine is approved, it will be much faster than the time normally required for approval of the vaccine, which will take place within 10 months of the genetic code being detailed, while the average wait for approval in the United States is about eight years.
Registration for emergency use was “a milestone in our journey to deliver the covid-19 vaccine to the world,”Pfizer chief executive Albert Borla said Thursday.
The first doses will be limited, and the Centers for Disease Control and Prevention will decide who has priority access to them.
The president of the European Commission, Ursula von der Leyen, said that the EU could also move quickly, by the end of the year.
But there are caveats, with Stephen Evans, professor of pharmacological epidemiology at the London School of Hygiene and Tropical Medicine, saying that both the food and Drug Administration and the European Medicines Agency will make a “very careful assessment.”
The BBC’s health correspondent, Naomi Grimley, said this vaccine is still far from widespread use, in part because it adopts an experimental technique that has not been approved before.
How effective is the vaccine?
Data released this week indicated that the Pfizer and biontek vaccine is 95 percent effective.
This effectiveness is consistent across age groups, races and sex.
With side effects mild to moderate and short-term.
The vaccine uses an experimental technique that involves injecting part of the virus’s genetic code into the body to train the immune system.
The body then makes antibodies to fight coronavirus.
What are the latest covid-19 developments in the United States?
This week, the United States passed the 250,000 deaths from the Coronavirus outbreak, the largest in the world.
The number of confirmed cases since the epidemic began has reached 11.7 million, according to Johns Hopkins University research.
Confirmed cases and deaths have also risen over the past week.
The health system in many areas of the United States is struggling to cope with the outbreak, with the establishment of temporary wards and wards in hospitals.
Issued by the Centers for Disease Control and Prevention a “strong recommendation” that Americans travel during the Thanksgiving holiday.
From Saturday, the state of California will impose a curfew for the vast majority of the population from 22:00 to 05:00.
Ohio, Minnesota and New York impose strict restrictions.
What other vaccines are being developed?
Available data indicate a vaccine produced by the American company Moderna that provides almost 95% protection, and Moderna uses the same approach as Pfizer.
The Oxford University/ AstraZeneca vaccine is still in trials but has shown promising results, with a strong immune response in the elderly. Britain ordered 100 million doses of it.
The Oxford/ AstraZeneca vaccine is produced from a weakened version of the common cold virus from chimpanzees, which has been modified so that it does not grow in humans.
There are also similarly promising results from the trials of another vaccine developed in Russia, called Sputnik. It works according to the Oxford Vaccine curriculum.
Other vaccines are in late test stages in China and Russia.